The Cell-Check™ Viability/Cytotoxicity Kit for Animal Cells provides a two-color fluorescence staining on both live and dead cells using two probes that measure two recognized parameters of cell viability — intracellular esterase activity and plasma membrane integrity. The kit is suitable for use with fluorescence microscopes, fluorescence multiwell plate scanners and flow cytometers and other fluorescence detection systems. The assay principles are general and applicable to most eukaryotic cell types, including adherent cells and certain tissues, but not to bacteria or yeast. It is generally faster, less expensive, safer and a more sensitive indicator of cytotoxic events than alternative methods.
Features:
Cell viability assay
I am doing a Cell-Check™ assay using Calcein, AM, for live cells and PI for dead cells. Can I fix the cells after labeling and retain the staining?
I need to use a dead cell control for my viability assay. Do you have a protocol for killing cells for this?
Antibacterial effect of bacteriocin XJS01 and its application as antibiofilm agents to treat multidrug-resistant Staphylococcus aureus infection
A novel bacteriocin against Staphylococcus aureus from Lactobacillus paracasei isolated from Yunnan traditional fermented yogurt: Purification, antibacterial characterization, and antibiofilm activity
Antibacterial activity and action target of phenyllactic acid against Staphylococcus aureus and its application in skim milk and cheese
Purification, characterization, and antibacterial and antibiofilm activity of a novel bacteriocin against Salmonella Enteritidis.
A Novel Bacteriocin Against Shigella flexneri From Lactiplantibacillus plantarum Isolated From Tilapia Intestine: Purification, Antibacterial Properties and Antibiofilm Activity.
Effect of surface sealant on surface roughness and bacterial adhesion of bulk-fill composites
Antibacterial activity and mechanism of action of bacteriocin LFX01 against Staphylococcus aureus and Escherichia coli and its application on pork model
Streptococcus Mutans adhesion to dental restorative materials after polishing with varioussystems:AConfocal Microscopy study
A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: Isolation, purification, identification, antibacterial and antibiofilm activity
Live cells are distinguished by the presence of ubiquitous intracellular esterase activity, determined by the enzymatic conversion of the virtually nonfluorescent cell-permeant calcein AM to the intensely fluorescent calcein. The polyanionic dye calcein is well retained within live cells, producing an intense uniform green fluorescence in live cells (Ex/Em ~495 nm/~520 nm). Propidium iodide (PI) enters cells with damaged membranes and undergoes a 30-fold enhancement of fluorescence upon binding to nucleic acids, thereby producing a bright red fluorescence in dead cells (Ex/Em ~528 nm/~617 nm). PI is excluded by the intact plasma membrane of live cells.
Specifications
Platform:
Fluorescence Microscopy, Flow Cytometry
Detection Method:
Fluorescent
Ex/Em:
Calcein: 494/517; PI: 535/617 nm
Product Size:
1000 assays
Storage Conditions:
-20 ℃, protect from light
Shipping Condition:
Ice pack
Applications
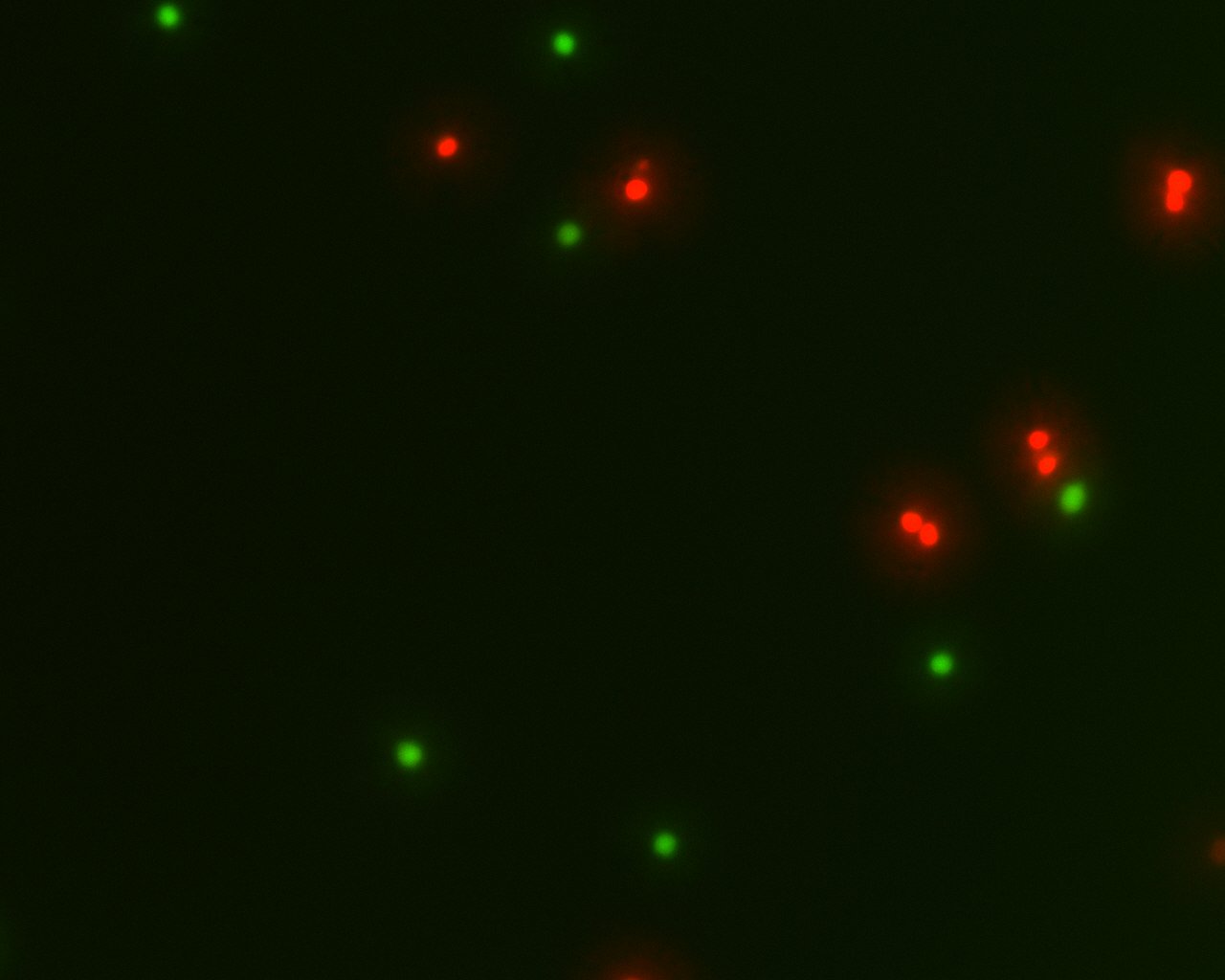
Figure 1. Live and dead Jurkat cells stained with Cell-Check™ Viability/Cytotoxicity Kit (A017). Live cells fluoresce a bright green, whereas dead cells fluoresce red.
Protocol (PDF):
A017
MSDS (PDF):
Component A
Component B
COA (PDF):
COA-A017
Frequently asked questions (FAQs)
This is not recommended. Neither Calcein nor PI bind to any cellular components upon fixation. There is no guarantee that the dyes will be retained upon fixation or any subsequent wash steps. We recommend scoring for live and dead cells as soon as possible after staining.
Heat killing is commonly used. Place your cells in a tube in buffer and heat at 60oC for 20 minutes. You can also kill your cells by fixing them with ice cold 70% ethanol for 15 minutes. The ethanol-killed cells can then be stored at -20oC until needed, at which point you wash out the ethanol and replace with buffer.
Cited Reference:
Yi-Zhou Xiang, Gang Wu, Lin-Yu Yang, Xiao-Jie Yang, Yan-Mei Zhang, Lian-Bing Lin, Xian-Yu Deng, Qi-Lin Zhang
International Journal of Biological Macromolecules, 2022, 196, 13-22, https://doi.org/10.1016/j.ijbiomac.2021.11.136
Yu-Hang Jiang, Wei-Gang Xin, Lin-Yu Yang, Jian-Ping Ying, Zi-Shun Zhao, Lian-Bing Lin, Xiu-Zhang Li, Qi-Lin Zhang
Journal of Dairy Science, 2022, 105(3):2094-2107. https://doi.org/10.3168/jds.2021-21126
Yu-Hang Jiang, Jian-Ping Ying, Wei-Gang Xin, Lin-Yu Yang, Xiu-Zhang Li, Qi-Lin Zhang
J. Dairy Sci. 2022, 105:9463–9475. https://doi.org/10.3168/jds.2022-22262
Xiang, Y.-Z., Zhang, Y.-M., Liu, Y.-Y., Zhang, M., Lin, L.-B., & Zhang, Q.-L.
Food Control, 2021, 127, 108110. doi:10.1016/j.foodcont.2021.10
Jiang Y-H, Xin W-G, Zhang Q-L, Lin L-B and Deng X-Y
Front. Microbiol. 2022, 12:779315. doi: 10.3389/fmicb.2021.779315
Gunce Ozan, Meltem Mert Eren, Cansu Vatansever and Ugur Erdemir
Polymers and Polymer Composites, 2021, Vol. 29(9S) S475–S484. DOI: 10.1177/09673911211005586
Wei-Gang Xin, Gang Wu, Jian-Ping Ying, Yi-Zhou Xiang, Yu-Hang Jiang, Xian-Yu Deng, Lian-Bing Lin, Qi-Lin Zhang
Meat Science, 2023, 196, 109045, https://doi.org/10.1016/j.meatsci.2022.109045
M.MertErena,G.Ozanb,U.Erdemirb,C.Vatansever
Acta Microscópica, 2021, 30, 1, 53-64
Hong-Wei Li, Yi-Zhou Xiang, Man Zhang, Yu-Hang Jiang, Yao Zhang, Ying-Yang Liu, Lian-Bing Lin, Qi-Lin Zhang
LWT, 2021, 140, 110826, https://doi.org/10.1016/j.lwt.2020.110826
Home » Cell Proliferation and Viability » Cell-Check™ Viability/Cytotoxicity Kit for Animal Cells
Cell-Check™ Viability/Cytotoxicity Kit for Animal Cells
Introduction
To order
Documents
Contact Us
ABP Biosciences
405 E Gude Dr, STE 214
Rockville, MD 20850
Service Hotline
Tel: 301-658-7993
E-mail: info@abpbio.com
Copyright@ 2026 ABP Biosciences, LLC. All Rights Reserved.

