Click chemistry describes a class of chemical reactions that use bio-orthogonal or biologically unique moieties to label and detect a molecule of interest in mild, aqueous conditions. The click reaction involves a copper-catalyzed triazole formation from an azide and an alkyne. The azide and alkyne moieties can be used interchangeably; either one can be used to tag the molecule of interest, while the other is used for subsequent detection.
Reference
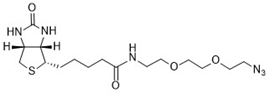
The biotin azide is reactive with terminal alkyne via a copper-catalyzed click reaction. Biotin can be subsequently detected with streptavidin, avidin or NeutrAvidin® biotin-binding protein.
Features
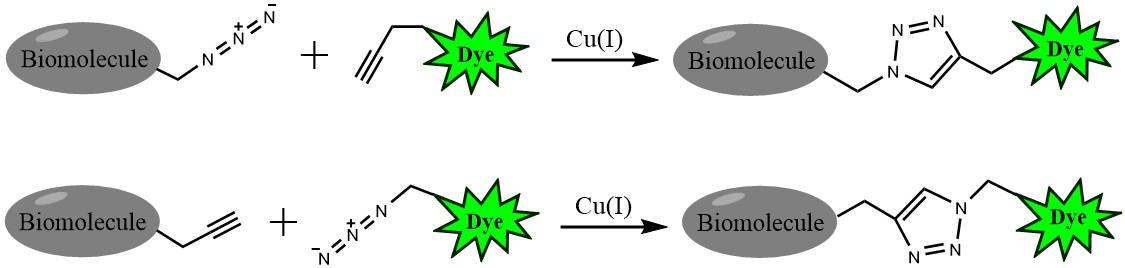
Figure 1. Click chemistry labeling
Specifications
Label:
Biotin
Ex/Em:
–
Detection Method:
–
Solubility:
DMSO, DMF
Molecular Weight:
400.50
Product Size:
5 mg
Storage Conditions:
-20 ℃, protect from light
Shipping Condition:
Room Temperature
Applications
Click chemistry labeling
Datasheet (PDF):
C304
MSDS (PDF):
C304
J Am Chem Soc (2008) 130:11576-11577
J Am Chem Soc (2009) 131:4967-4975
Chem Res Toxicol (2008) 21:432-444
Proc Natl Acad Sci U S A (2007) 104:2614-2619
Angew Chem Int Ed Engl (2009) 48:4030-4033
Home » Click Chemistry Tools » Biotin Azide
Biotin Azide
Introduction
To order
Documents
Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, Agnew BJ, Hsieh-Wilson LC,
Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC,
Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ,
Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH
Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu T, Bertozzi CR, Wu P
Contact Us
ABP Biosciences
405 E Gude Dr, STE 214
Rockville, MD 20850
Service Hotline
Tel: 301-658-7993
E-mail: info@abpbio.com
Copyright@ 2026 ABP Biosciences, LLC. All Rights Reserved.

